Pathologist’s Corner – Dr. Hon on Breast Cancer Tissue Handling and Updates for Her-2 Testing
Published: January 05, 2015 – Janet O. Hon, MD, FCAP
 Based on the 2013 ASCP/CAP Guidelines for HER2 Testing in Breast Cancer
Based on the 2013 ASCP/CAP Guidelines for HER2 Testing in Breast Cancer
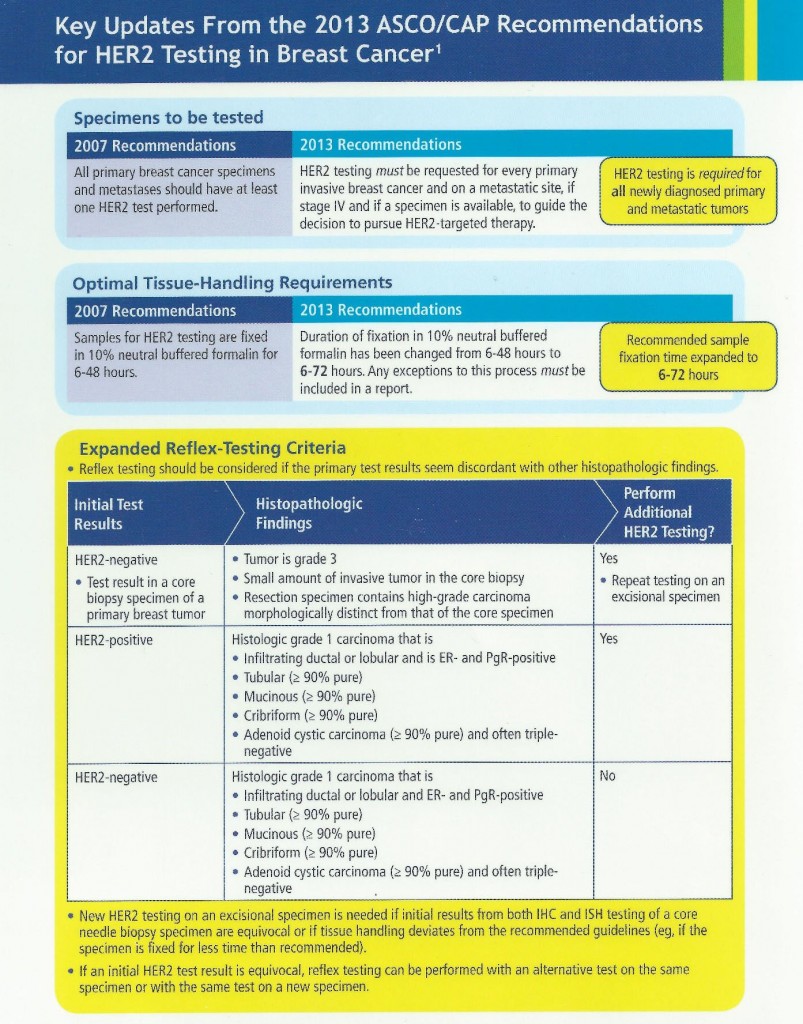
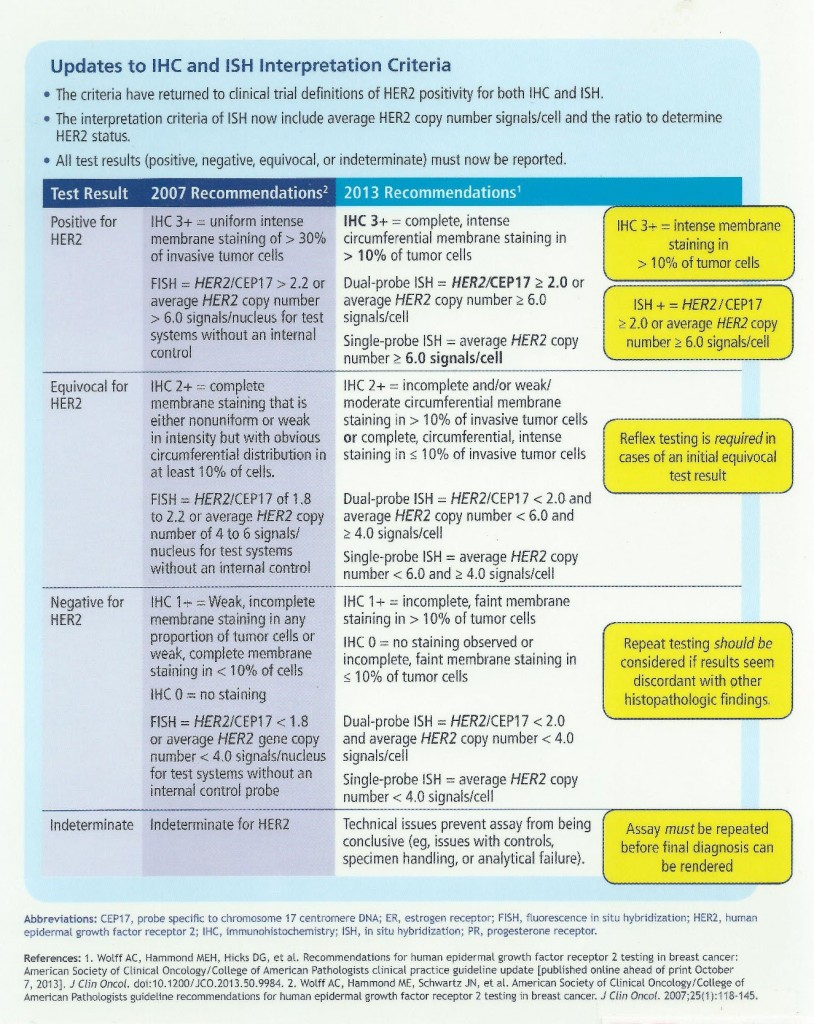
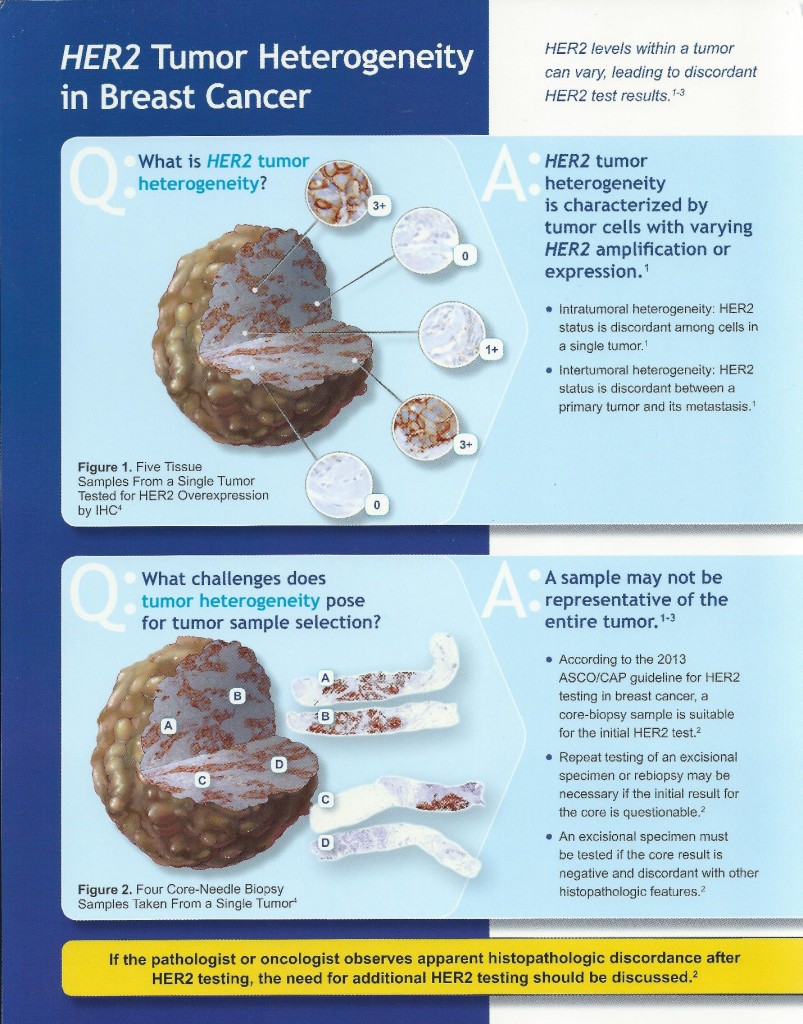
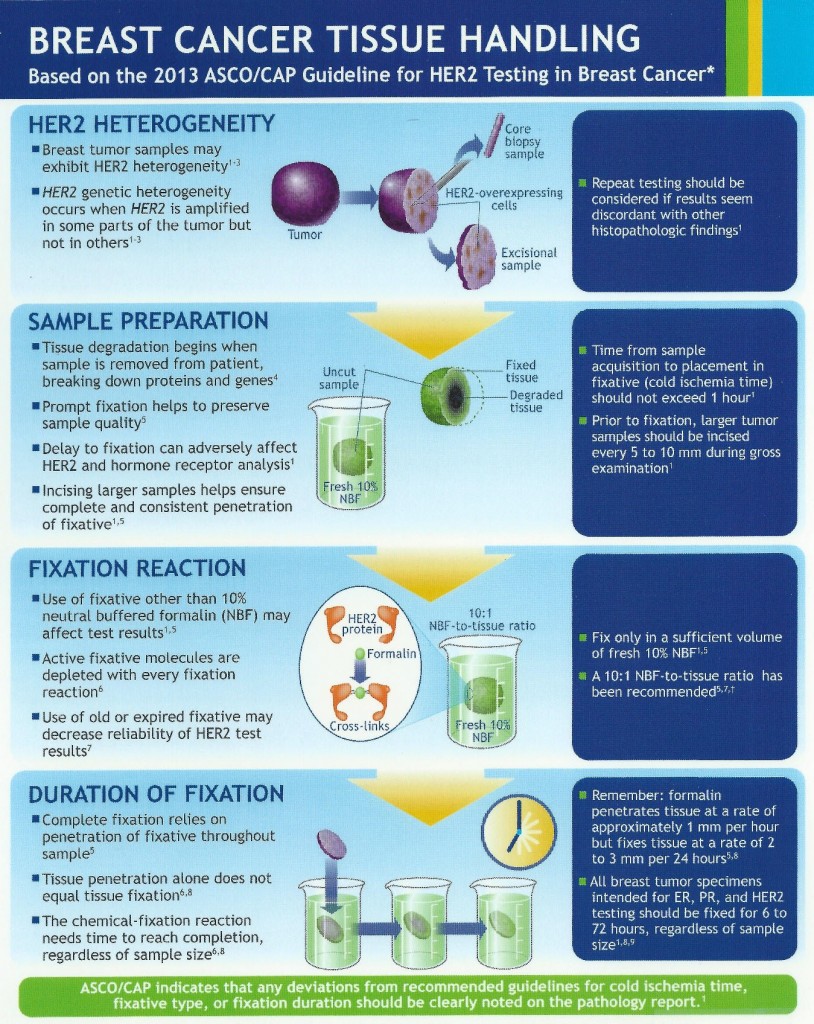
The introduction of breast bio-markers, in particular, estrogen and progesterone receptors and Her-2/neu, into clinical practice and their critically important role in adjuvant treatment decisions has created challenges for the surgical pathology laboratory. Initially, frozen tissue containing tumor was sent for estrogen receptor analysis in the 1980s. With the advent of receptor testing on formalin fixed, paraffin embedded tumor tissue in the early 1990s, the standards for collection and preservation of clinical samples focused on tissue preservation for morphologic examination, with little if any attention paid to preserving the quality of macromolecules that may be in the tissue. Because of the importance of these markers for determining the most appropriate treatments available for each patient, there was a need for standardizing pre-analytic variables, with the goal of developing standardized methods of tissue procurement and processing, and documenting how these variables affect the quality of tissue for biomarker testing and molecular analysis. By better defining specimen handling requirements and approaching diagnostic tissue samples as analytes, we can improve the quality of routine diagnostic samples, which in turn will enhance adjuvant treatment decisions when dealing with breast cancer and other solid tumor malignancies. In view of this, ASCO and CAP developed guidelines for breast cancer tissue handling and evaluation of receptors and Her-2 in 2007. These guidelines were updated in 2013. Her-2 in particular can be heterogeneous in tumor tissue which requires additional considerations in sampling and evaluation. The updated ASCO/CAP guidelines and issues regarding Her-2 sampling are summarized in the following pictorials.
*The illustrations were kindly provided by Genentech for educational purposes.